In 1859 Gustav KirchhoffRobert Kirchhoff (1824–1887) was a physicist. His work with Robert Bunsen led to the discovery of spectral analysis. Kirchhoff also conducted research into electricity and was the first to describe the dependent nature of electrical current, electrical voltage, and electrical resistance. and Robert BunsenRobert Bunsen (1811–1899) was a chemist. His work with Robert Kirchhoff led to the discovery of spectral analysis and the elements caesium and rubidium. He perfected the Bunsen burner, which was named after him. discovered that every chemical element in the light spectrum has a unique “signature”.
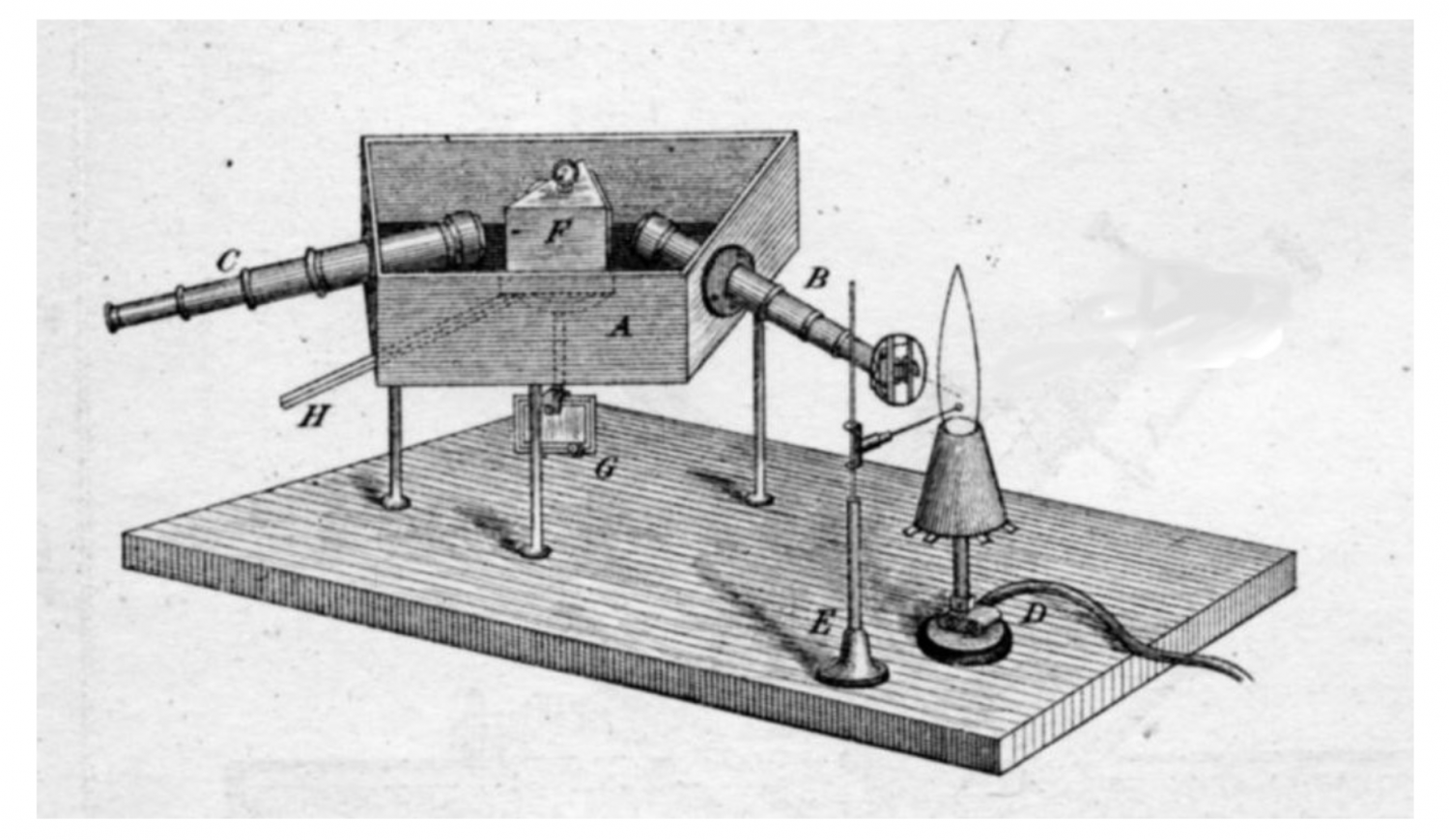
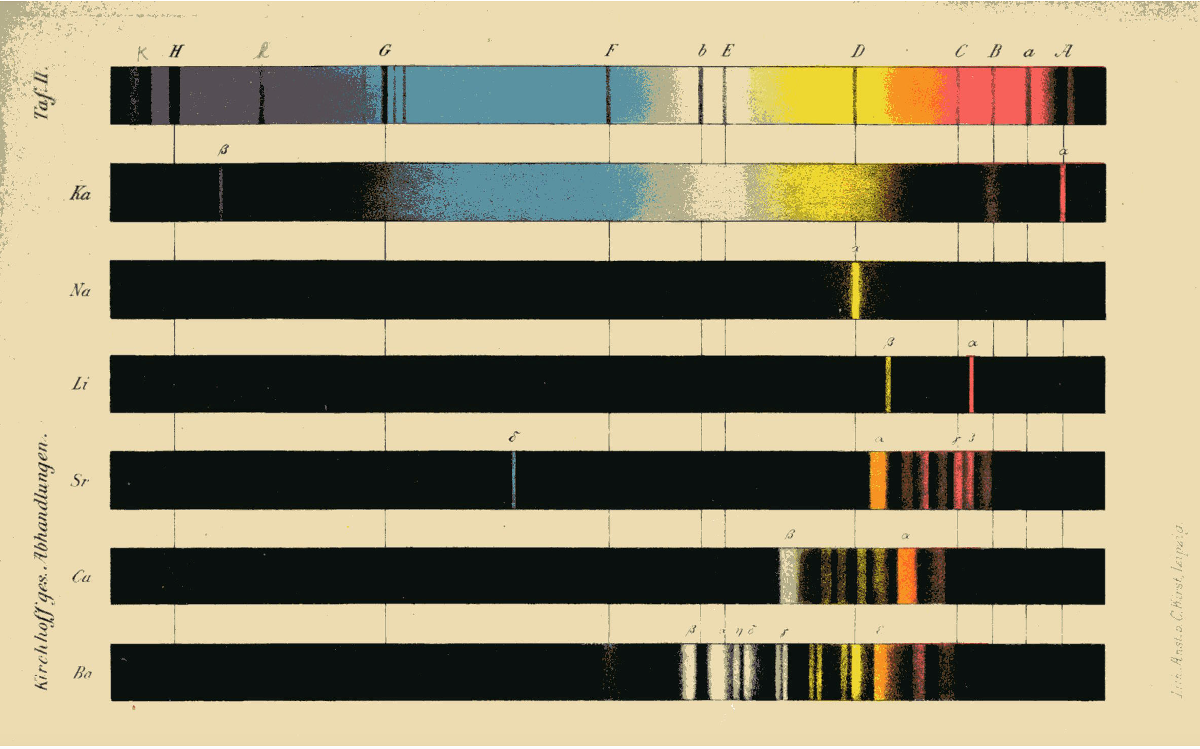
In their spectral analysis, Bunsen and Kirchhoff built on the discovery that Joseph von FraunhoferJoseph von Fraunhofer (1787–1826), optician and physicist, was a builder of telescopes and discovered the Fraunhofer lines – the absorption lines in the solar spectrum – which are named after him. He combined scientific precision with practical application and invented new instruments. On the basis of this, he also gave his name to the Fraunhofer Society. had made in 1813: that there are dark lines in the spectrum of the Sun and other stars. Bunsen and Kirchhoff now ascertained that these dark lines can be explained by the fact that particular chemical elements absorb particular wavelengths of light. In other words, if you look at the sunlight through a prism, these elements “obscure” parts of the light. Besides these dark absorption lines, there are also bright lines in the spectrum of stars. These occur when an associated chemical element is excited and emits light of a certain wavelength. By analysing these emission lines together with the absorption lines, it is possible to determine the chemical elements a star is composed of, even if it is light years away. All at once, it became clear that the same chemical elements that are found on Earth also occur on the Sun and other stars.
The spectral lines of the Sun and other stars were given added significance by the atomic model developed by Niels BohrNiels Bohr (1885–1962), physicist, made Albert Einstein’s acquaintance in Berlin in 1920. The atomic model he developed won him the Nobel Prize for Physics in 1922: it was based on the idea of light, negatively charged electrons moving in a closed orbit around a heavy, positively charged nucleus. His model anticipated elements of quantum mechanics – the assumption that electrons can “jump” from one orbit to another, causing the atom to emit radiation. in 1913. On the basis of Bohr’s model, a spectral line not only reveals the presence of a chemical element but also provides information about pressure and temperature on the star that is being observed.
Why was the discovery of spectral analysis and the spectrograph so important in the development of the Einstein Tower? Spectral analysis plays an essential role in the scientific experiments for which the tower was built.